
What’s in a Can? A Chat with Solvay about Surfactants
What is a Surfactant? A surfactant is a substance, that when added to water, reduces the surface tension of the solution thus increasing its substrate wetting properties. At low concentrations, a surfactant has the property of migrating and being..
What is a Surfactant?
A surfactant is a substance, that when added to water, reduces the surface tension of the solution thus increasing its substrate wetting properties. At low concentrations, a surfactant has the property of migrating and being adsorbed on the interfaces present in the system. It alters the interfacial free energies of these interfaces. Thus, a surfactant is a surface-active agent.Surfactants can be referred to by a variety of terms: soap, wetting agent, dispersing agents, substrate wetting additives, emulsifiers, surface-active agents, stabilizers, or solubilizers. They are necessary for the wet stage of polymers and coatings. Chemists use surfactants to change the composition of a liquid which enables specific properties that meet the needs for market applications. Since surfactants can also cause detrimental effects in the film, such as blisters, surfactant leaching, and less water resistance, chemists need to use the lowest levels possible while maintaining the coating’s performance.
Four Types of Surfactants
There are four basic classes of surfactants: nonionic, anionic, cationic, and amphoteric. All four types of surfactants can be utilized in pigment dispersions.
1. Nonionic Surfactants
Chemists use nonionic surfactants after polymerization to achieve stability, and then in the grind for wetting and dispersing pigments and again in the letdown to achieve optimal substrate wetting. Nonionic surfactants also help with stability of the formulation, heat age stability, freeze/thaw resistance, and in-can stability.
With nonionic surfactants, chemists should consider the HLB values as well as a cloud point. The HLB value plays a critical part in its functions and properties like emulsification, solubility, wetting, and dispersion. The cloud point affects storage conditions and if the cloud point is too low the coating may have phase separation and instability. For low-foam applications, the cloud point of the product should be just below the application temperature.
HLB (Hydrophile-Lipophile Balance)
HLB value is an empirical expression for the relationship of the hydrophilic ("water-loving") and hydrophobic ("water-hating") groups of a surfactant. The higher the HLB value, the more water-soluble (hydrophilic) the surfactant. The HLB value of nonionic surfactants is a measurement – on a scale of 1 to 20 – of the degree of water/oil solubility of a given surfactant.
The HLB of a particular surfactant is directly related to the structure of both its hydrophilic and hydrophobic parts and plays a critical role in its function and properties.
To calculate HLB, take the molecular weight of the surfactant hydrophilic chain, divided by the total molecular weight, and then multiply by 20.
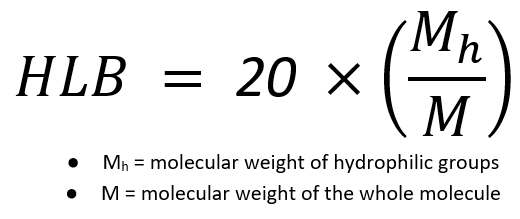
Cloud Point
The cloud point marks the temperature above which an aqueous solution of a water-soluble surfactant becomes cloudy or turbid in appearance. Cloud points range from 0° to 100°C (32 to 212°F), limited by the freezing and boiling points of water. The cloud point is directional for determining storage stability and utility of surfactants at different temperatures.
As you can see, these two characteristics, HLB and cloud point, can be highly influential in determining the effectiveness of selected surfactants for different applications.
2. Anionic Surfactants
Chemists use anionic surfactants during polymerization as primary and secondary emulsifiers. They are used to control electrostatic stabilization and particle size. Unlike their nonionic counterparts, anionic surfactants don’t have a theoretical HLB value or a cloud point.3. Cationic Surfactants
Chemists use cationic surfactants for their positive charge and anti-static properties. Typically, we see cationic surfactants used in textiles for their fabric softening ability and coatings for their anti-corrosive and anti-static properties.4. Amphoteric Surfactants
Amphoteric surfactants are naturally cationic at low pH and anionic at high pH. They are very mild and non-irritating when used in cleaners and detergents.Applications for Surfactants
To determine what surfactant to work with it is important to understand where, in what stage, will the surfactant be utilized and what performance is required.Polymer chemists and formulators in paints and coatings use surfactants at nearly every stage:
- Polymerization
- Emulsifier
- Stabilizer
- Post-polymerization additives
- Grind, wetting out the pigments so they can be dispersed
- Letdown
- Lowering the surface tension for wetting out substrates
- Formulation stability
- Other performance properties
As with any other raw material evaluation, it is important to determine if the desired outcome was achieved and were there any effects to the performance with the addition.
For additional information on surfactants, please contact
Joey Ruiz, Ph. D.
Principal Scientist, Solvay
(215)781-7895 Office
joey.ruiz@solvay.com

 Construction
Construction
 Nonwovens
Nonwovens
 Adhesives
Adhesives
 Textiles
Textiles
 Printing & Packaging
Printing & Packaging
 Paper
Paper
 Paints & Coatings
Paints & Coatings





